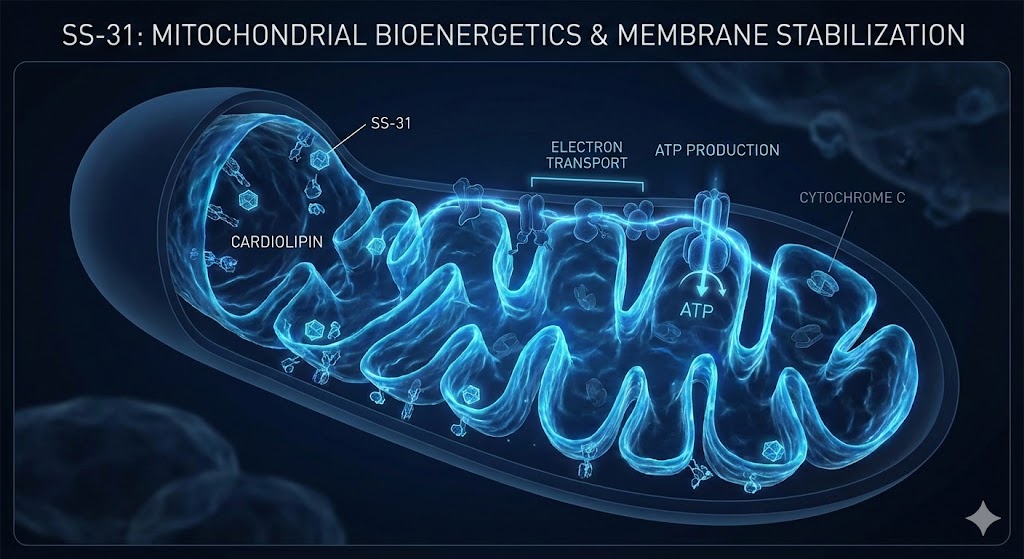
In the rapidly evolving field of mitochondrial medicine and peptide therapeutics, few compounds have generated as much investigative interest as SS-31 (elamipretide). As a member of the Szeto-Schiller (SS) family of peptides, SS-31 is distinct in its ability to target the inner mitochondrial membrane (IMM). Unlike general antioxidants that scavenge reactive oxygen species (ROS) throughout the cell, SS-31 is engineered to localize specifically to the source of oxidative stress.
For researchers utilizing the SPARX BIOTECH PEPTIDE platform, understanding the precise molecular interactions of SS-31 is critical for designing robust experiments in cellular bioenergetics, aging, and ischemia-reperfusion models.
The Structural Basis of Szeto-Schiller Peptides
SS-31 is a synthetic tetrapeptide with the sequence D-Arg-Dmt-Lys-Phe-NH2. Its design incorporates alternating aromatic and basic amino acid residues, a structural motif that allows the peptide to be cell-permeable and target mitochondria without the need for specific transporters or reliance on mitochondrial membrane potential.
Research indicates that SS-31 can concentrate in the mitochondrial matrix up to 1000-fold compared to the cytosol. This high specificity is driven by the peptide’s affinity for cardiolipin, a unique phospholipid component of the inner mitochondrial membrane. This interaction is the cornerstone of SS-31’s stabilizing effects and separates it from other antioxidant compounds that lack organelle specificity.
The Critical Role of Cardiolipin in Mitochondrial Health
To understand how SS-31 functions, one must first examine its primary target: cardiolipin (CL). Cardiolipin is an anionic phospholipid found almost exclusively in the inner mitochondrial membrane. It plays a structural and functional role that is vital for mitochondrial efficiency.
1. Cristae Architecture
Cardiolipin is essential for the formation and maintenance of cristae—the folds of the inner membrane where the electron transport chain (ETC) resides. These folds increase the surface area available for energy production. Loss of cardiolipin integrity leads to the flattening of cristae and a subsequent drop in bioenergetic capacity.
2. Supercomplex Assembly
Research suggests that cardiolipin acts as a 'glue' that stabilizes the respiratory supercomplexes (respirasomes). By organizing Complex I, III, and IV into tight clusters, cardiolipin facilitates the efficient transfer of electrons, minimizing electron leakage.
3. Proton Trap
The unique structure of cardiolipin allows it to trap protons on the outer surface of the inner membrane, effectively functioning as a proton buffer that aids in the operation of ATP synthase.
Mechanism of Action: Stabilizing the Membrane
In models of mitochondrial dysfunction—whether induced by age, metabolic stress, or ischemic injury—cardiolipin is often a primary casualty. Oxidative stress leads to the peroxidation of cardiolipin, which disrupts its ability to maintain membrane curvature and stabilize ETC complexes.
SS-31 interacts with cardiolipin via electrostatic and hydrophobic forces. This interaction provides several stabilizing benefits observed in preclinical research:
- Prevention of Cardiolipin Peroxidation: By binding to cardiolipin, SS-31 alters the molecular environment of the phospholipid, making the linoleic acid residues within cardiolipin less accessible to cytochrome c peroxidase activity. This prevents the oxidative degradation of the membrane architecture.
- Restoration of Electron Transport Efficiency: When SS-31 stabilizes cardiolipin, it supports the continued aggregation of ETC supercomplexes. Studies utilizing high-resolution respirometry have shown that this stabilization can improve the coupling of electron transport to oxidative phosphorylation (ATP production), even under conditions of stress.
- Inhibition of Cytochrome c Release: One of the hallmarks of severe mitochondrial stress is the release of cytochrome c into the cytosol, a key trigger for apoptosis. By stabilizing the interaction between cardiolipin and cytochrome c, SS-31 helps retain cytochrome c within the intermembrane space.
SS-31 and Oxidative Stress: A Targeted Approach
A common misconception in peptide research is categorizing SS-31 simply as an 'antioxidant.' While it reduces net oxidative stress, its mechanism is distinct from direct scavengers like Vitamin C or E.
Direct scavengers neutralize ROS after they are produced. However, high concentrations of ROS can damage cellular machinery before a scavenger can intervene. SS-31 operates upstream of this damage. By optimizing the efficiency of the electron transport chain, SS-31 reduces 'electron slip'—the leakage of electrons from the chain that react with oxygen to form superoxide radicals.
Consequently, research suggests SS-31 prevents the formation of ROS at the source rather than merely cleaning them up. This preservation of mitochondrial redox balance is critical in research focused on:
- Neuroprotection models: Investigating neuronal survival in hypoxic conditions.
- Renal and Cardiac physiology: Studying organ preservation during reperfusion injury.
- Skeletal Muscle aging: Analyzing mitochondrial coupling in senescent tissue.
Current Research Frontiers
The versatility of SS-31 has led to its inclusion in a wide array of research protocols. Current investigations are exploring the peptide's utility in:
- Metabolic Syndrome Models: Examining how mitochondrial membrane stabilization affects insulin sensitivity and lipid oxidation in adipocytes.
- Inflammation: Research indicates that mitochondrial ROS is a trigger for the NLRP3 inflammasome. SS-31 is being used to study pathways linking mitochondrial health to systemic inflammation.
- Longevity Science: Given the central role of mitochondrial decline in aging theories, SS-31 is a primary tool for studying the effects of membrane preservation on lifespan and healthspan in model organisms.
Related Research
Explore further research on mitochondrial peptides and tissue repair:
Conclusion
SS-31 represents a sophisticated approach to studying mitochondrial bioenergetics. By targeting the structural integrity of the inner mitochondrial membrane through cardiolipin interaction, it offers researchers a unique method to modulate oxidative stress and energy production at the organelle level. As the scientific community continues to unravel the complexities of mitochondrial dynamics, peptides like SS-31 remain essential tools for defining the boundaries of cellular resilience.
SPARX BIOTECH PEPTIDE remains committed to supplying high-purity, research-grade peptides to support these critical investigations.