Introduction to GLP-1 Receptor Agonists in Research
Semaglutide is a modified analogue of the endogenous peptide hormone Glucagon-Like Peptide-1 (GLP-1). In biotechnology and metabolic research, it serves as a critical tool for investigating the physiological pathways governing energy homeostasis, glucose metabolism, and appetite regulation. Unlike endogenous GLP-1, which has a half-life of only minutes due to rapid degradation by the enzyme dipeptidyl peptidase-4 (DPP-4), Semaglutide has been structurally modified to resist enzymatic cleavage, allowing for sustained receptor activation in experimental models.
The primary focus of current investigation involves the interaction between Semaglutide and the GLP-1 receptor (GLP-1R), a Class B G-protein-coupled receptor (GPCR). This interaction triggers a cascade of intracellular signaling events that have been widely studied in preclinical models of obesity and metabolic dysfunction. This article examines the molecular mechanisms by which GLP-1 signaling influences appetite control circuits, focusing on hypothalamic integration and the gut-brain axis.
Molecular Mechanism of GLP-1 Receptor Activation
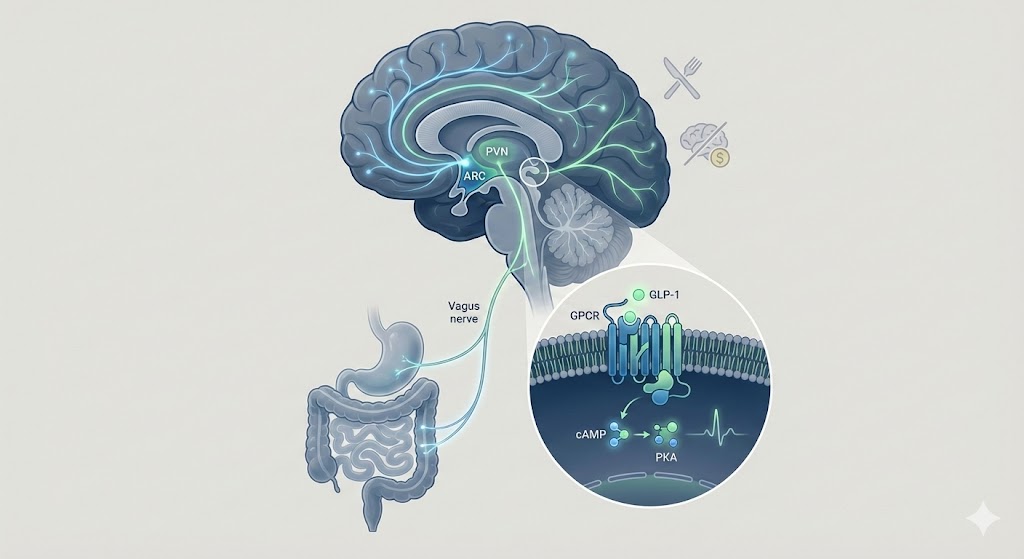
The GLP-1 receptor is widely expressed in pancreatic beta cells, the gastrointestinal tract, and specific regions of the central nervous system (CNS). Research indicates that Semaglutide functions as a potent agonist, binding to the extracellular domain of the GLP-1R with high affinity.
The cAMP-PKA Signaling Cascade
Upon binding, the GLP-1 receptor undergoes a conformational change that activates the heterotrimeric G-protein complex, specifically the Gs alpha subunit (Gαs). This activation initiates a well-documented signaling cascade:
- Adenylate Cyclase Activation: The activated Gαs subunit stimulates adenylate cyclase, leading to the conversion of ATP into cyclic adenosine monophosphate (cAMP).
- PKA Activation: Elevated intracellular cAMP levels activate Protein Kinase A (PKA) and Exchange Protein Directly Activated by cAMP (EPAC).
- Downstream Effects: In pancreatic research models, this pathway is linked to the closure of ATP-sensitive potassium channels (KATP) and the opening of voltage-dependent calcium channels (VDCCs), resulting in calcium influx and insulin granule exocytosis.
However, in the context of appetite control, research focuses on how this signaling pathway modulates neuronal activity within the brain's feeding centers.
Central Nervous System Regulation of Appetite
Preclinical studies suggest that the profound anorexigenic (appetite-suppressing) effects observed in Semaglutide research are primarily mediated through direct modulation of the Central Nervous System.
Hypothalamic Nuclei Involvement
The hypothalamus is the central integrator of energy status. Research utilizing fluorescently labeled GLP-1 agonists has demonstrated that these peptides can cross the blood-brain barrier (BBB) or access the brain via the circumventricular organs (CVOs), such as the median eminence and the area postrema, which lack a complete BBB.
- Arcuate Nucleus (ARC): The ARC contains two distinct populations of neurons: the appetite-stimulating (orexigenic) NPY/AgRP neurons and the appetite-suppressing (anorexigenic) POMC/CART neurons. Studies suggest that GLP-1 receptor activation directly stimulates POMC neurons while indirectly inhibiting NPY/AgRP neurons via GABAergic interneurons.
- Paraventricular Nucleus (PVN): Downstream signaling from the ARC projects to the PVN, where GLP-1 signaling has been shown to modulate neurons involved in satiety perception.
Brainstem and Hindbrain Activation
Beyond the hypothalamus, the solitary tract nucleus (NTS) in the brainstem is a critical site of action. The NTS receives vagal afferent signals from the gut and integrates them with descending hypothalamic inputs. Research indicates that GLP-1R activation in the hindbrain enhances the response to satiety signals, effectively amplifying the "fullness" message sent from the gastrointestinal tract during nutrient intake.
The Gut-Brain Axis and Peripheral Signaling
While CNS penetration is a key area of study, peripheral mechanisms also play a significant role in appetite control protocols.
Vagal Nerve Stimulation
The gastrointestinal tract is densely innervated by the vagus nerve, which expresses GLP-1 receptors on its afferent fibers. In animal models, activation of these receptors by GLP-1 agonists generates signals that are transmitted to the NTS. This "bottom-up" signaling pathway is believed to mediate the reduction in meal size and the delay in gastric emptying observed in metabolic studies.
Gastric Motility and Nutrient Absorption
Research has consistently shown that GLP-1 signaling slows gastric emptying. By retaining nutrient content in the stomach for longer periods, mechanical stretch receptors remain activated. In experimental settings, this delay has been correlated with prolonged post-prandial satiety signals, reducing the frequency of feeding behaviors in rodent models.
Reward Pathways and Hedonic Feeding
Recent investigations have expanded beyond homeostatic feeding (eating for energy) to hedonic feeding (eating for pleasure). This involves the mesolimbic dopamine system, specifically the Ventral Tegmental Area (VTA) and the Nucleus Accumbens (NAc).
Preclinical data suggests that GLP-1 receptors are expressed in these reward-processing regions. Studies involving Semaglutide administration in rodents have documented a reduction in the motivation to work for palatable food rewards (high-fat or high-sugar pellets). This implies that GLP-1 signaling may dampen the rewarding value of food, potentially by modulating dopamine turnover in the VTA.
Future Directions in Peptide Research
The success of Semaglutide in research settings has catalyzed interest in next-generation peptide engineering. While Semaglutide focuses on satiety, other peptides are being investigated for complementary metabolic effects:
- Mitochondrial Metabolism: Research into MOTS-c and mitochondrial metabolism explores how regulating cellular energy flux can impact metabolic flexibility alongside appetite control.
- Tissue Regeneration: While GLP-1 targets the pancreas and brain, peptides like GHK-Cu are studied for tissue regeneration properties, highlighting the diverse applications of peptide synthesis.
- Systemic Repair: Comparative studies often look at BPC-157 repair models to understand how different signaling pathways influence healing versus metabolic regulation.
Current areas of investigation also include:
- Dual Agonists: Molecules that co-activate GLP-1 and GIP (Glucose-dependent Insulinotropic Polypeptide) receptors to investigate synergistic effects on metabolic efficiency.
- Triple Agonists: "Tri-agonists" targeting GLP-1, GIP, and Glucagon receptors, aiming to simultaneously suppress appetite and increase energy expenditure in obese phenotypes.
- Neuroprotection: Emerging research is exploring the potential neuroprotective properties of GLP-1 signaling in models of neurodegenerative disorders, given the high density of receptors in the hippocampus and cortex.
Conclusion
Semaglutide remains a cornerstone compound in metabolic research, offering deep insights into the complex neuroendocrine pathways governing appetite. By activating GLP-1 receptors in both the CNS and the periphery, it modulates a diverse array of physiological processes—from hypothalamic signaling to gastric motility. As biotechnology advances, the continued study of these pathways promises to refine our understanding of energy homeostasis.