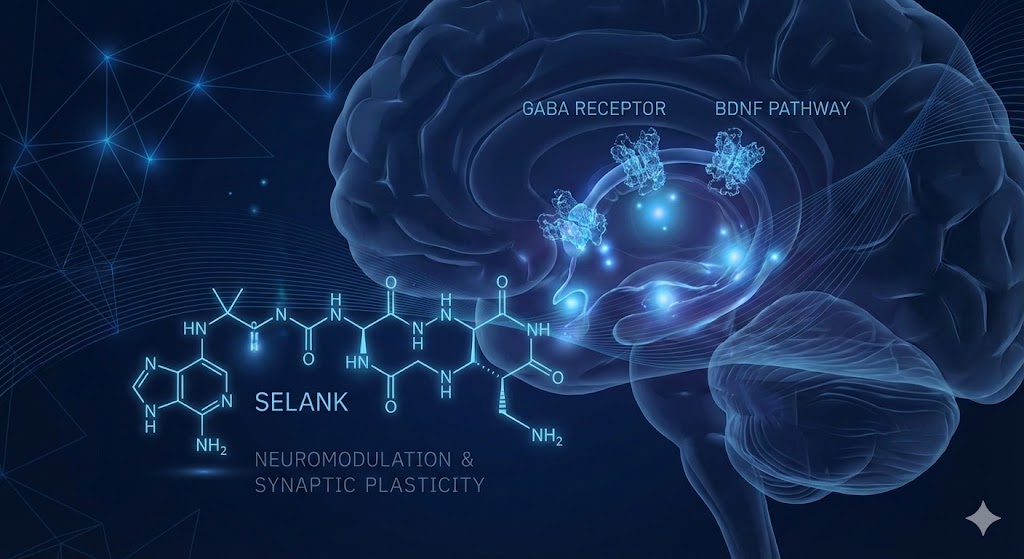
The exploration of synthetic regulatory peptides has opened new avenues in neurobiology, particularly regarding the modulation of the central nervous system (CNS) without the sedative side effects often associated with traditional pharmacological agents. Among these, Selank peptide has emerged as a significant subject of investigation.
A synthetic analog of the immunomodulatory peptide tuftsin, Selank (Thr-Lys-Pro-Arg-Pro-Gly-Pro) is characterized by its unique ability to influence the GABAergic system and neurotrophic factors simultaneously. This article reviews the current scientific understanding of Selank’s mechanism of action, focusing on its interaction with GABA receptors, its structural stability, and its downstream effects on Brain-Derived Neurotrophic Factor (BDNF).
The Structural Basis: From Tuftsin to Selank
To understand the pharmacodynamics of Selank, one must first examine its structural origin. Selank is a heptapeptide derivative of tuftsin, a naturally occurring tetrapeptide (Thr-Lys-Pro-Arg) found in the heavy chain of human immunoglobulin G (IgG).
While tuftsin plays a vital role in immune function, its natural half-life is exceedingly short due to rapid enzymatic degradation. Selank was engineered by extending the C-terminus of tuftsin with a tripeptide sequence: Pro-Gly-Pro (PGP).
This structural modification serves two primary functions in research settings:
- Metabolic Stability: The PGP tail renders the peptide resistant to primary peptidases, significantly extending its half-life and bioavailability in biological models compared to endogenous tuftsin.
- Blood-Brain Barrier Penetration: The modification facilitates the transport of the peptide across the blood-brain barrier, allowing for direct interaction with CNS targets.
Mechanism of Action: The GABAergic System
The primary focus of Selank peptide research lies in its interaction with the gamma-aminobutyric acid (GABA) system, the principal inhibitory neurotransmitter system in the mammalian brain.
Allosteric Modulation vs. Direct Agonism
Unlike traditional anxiolytics (such as benzodiazepines) which act as direct agonists or positive allosteric modulators binding to specific subunits of the GABA(A) receptor, Selank appears to operate through a distinct mechanism.
Research suggests that Selank acts as a regulatory peptide that modulates the GABAergic system allosterically but does not bind to the benzodiazepine site directly. In electrophysiological studies and radioligand binding assays, Selank has been observed to change the affinity of the receptor for endogenous GABA, thereby enhancing inhibitory signaling without inducing the sedation, amnesia, or motor impairment often seen with direct receptor agonists.
Gene Expression Profiling
Transcriptomic analyses in rodent models have provided deeper insight into this modulation. Studies indicate that Selank administration can alter the mRNA expression levels of genes encoding:
- GABA receptor subunits.
- GABA transporters (GAT).
- Ion channels associated with neurotransmission.
This data suggests that Selank does not merely "activate" a receptor but rather induces a systemic shift in the expression and sensitivity of the GABAergic machinery, potentially restoring homeostatic balance in models of stress-induced neurochemistry.
Beyond GABA: BDNF Expression in the Hippocampus
While GABA receptor modulation explains the anxiolytic-like effects observed in preclinical trials, it does not fully account for the peptide's reported cognitive and neuroprotective properties. Further investigation has identified a secondary, crucial mechanism: the upregulation of neurotrophins. Similar regenerative mechanisms are often explored in studies involving GHK-Cu and tissue regeneration.
Rapid Elevation of BDNF
Brain-Derived Neurotrophic Factor (BDNF) is a protein critical for the survival of existing neurons and the growth and differentiation of new neurons and synapses (neuroplasticity).
In rat models, intranasal administration of Selank was found to induce a rapid increase in BDNF expression within the hippocampus. This effect is significant for two reasons:
- Neuroplasticity: The hippocampus is the center of memory and learning. Elevated BDNF levels are correlated with improved long-term potentiation (LTP) and memory consolidation.
- Stress Resilience: Chronic stress is known to deplete hippocampal BDNF. By counteracting this depletion, Selank is hypothesized to protect neural circuitry from stress-induced remodeling.
Inhibition of Enkephalin-Degrading Enzymes
A third pillar of Selank’s mechanism involves the endogenous opioid system. Enkephalins are peptides involved in regulating nociception (pain) and stress responses. They are typically short-lived, degraded rapidly by enzymes such as enkephalinase.
Biochemical assays have demonstrated that Selank inhibits the activity of enkephalin-degrading enzymes in human serum. By slowing the breakdown of endogenous enkephalins, Selank indirectly prolongs their physiological activity. This preservation of enkephalins may contribute to the peptide's ability to modulate stress responses and stabilize mood in animal subjects, acting synergistically with its GABAergic effects.
Summary of Research Applications
The unique pharmacological profile of Selank—combining GABA modulation, BDNF upregulation, and enkephalinase inhibition—positions it as a versatile tool in biotechnology research. Researchers investigating systemic repair models may also be interested in the comparative effects found in BPC-157 repair models.
Current areas of investigation include:
- Anxiety and Stress Models: Evaluating the peptide's efficacy in reducing anxiety-like behaviors in "open field" and "elevated plus maze" tests.
- Cognitive Decline: Investigating the potential of Selank to mitigate memory impairment in models of neurodegeneration or ethanol-induced toxicity.
- Immunomodulation: Studying the peptide’s influence on Interleukin-6 (IL-6) and the balance of T-helper cytokines, reflecting its tuftsin heritage.
Conclusion
Selank peptide represents a sophisticated example of rational drug design, improving upon a natural immunopeptide to create a stable, CNS-active compound. Its mechanism is multifaceted, moving beyond simple receptor agonism to include the allosteric modulation of the GABA receptor complex and the upregulation of critical neurotrophic factors like BDNF.
As research continues, Selank remains a compound of high interest for understanding the biochemical intersection of anxiety regulation, cognitive maintenance, and peptide therapeutics. For further reading on mitochondrial metabolism in peptide research, see our analysis of MOTS-c.