Primary Keyword: Oxytocin signaling in sexual arousal
Secondary Keywords: Neurobiology of sexual behavior, Oxytocin receptor (OXTR) pathways, Hypothalamic peptide regulation, Dopamine-oxytocin interaction, Peptide research models
Introduction to Oxytocin Neuropeptide Research
Oxytocin (OXT) is a nonapeptide synthesized primarily in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus. While classically recognized for its role in parturition and lactation, extensive neurobiological research has established OXT as a critical modulator of complex social behaviors and sexual reflexes.
In the context of peptide research, oxytocin remains a focal point for investigating the central mechanisms of sexual arousal and response. Current literature focuses on delineating the precise neural circuitry through which OXT influences sexual motivation, penile erection, and lordosis behavior in various animal models. This article explores the biochemical pathways and receptor interactions that characterize oxytocin’s role in sexual physiology.
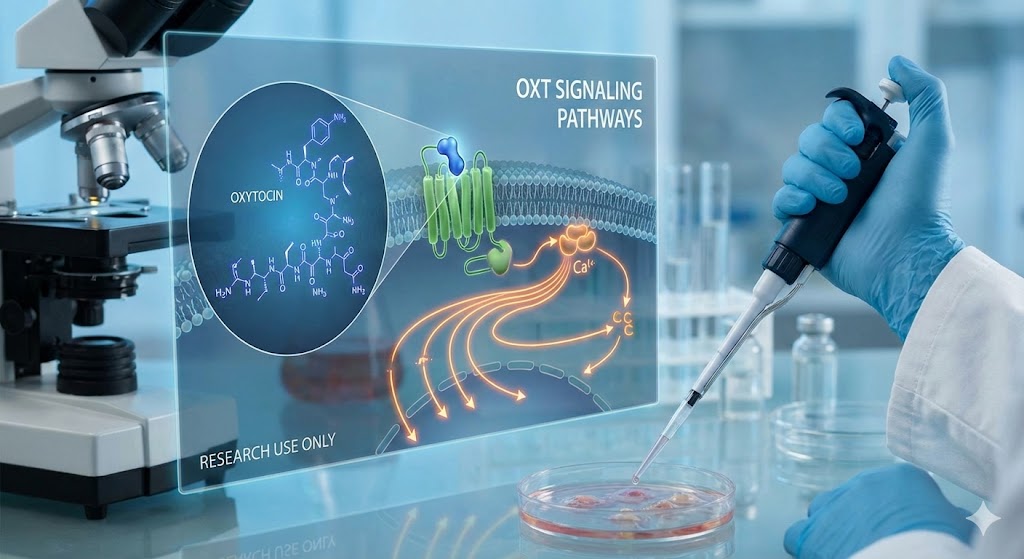
The Oxytocin Receptor (OXTR) and Intracellular Signaling
The physiological effects of oxytocin are mediated through the oxytocin receptor (OXTR), a G-protein-coupled receptor (GPCR). Research indicates that the distribution of OXTRs in the central nervous system is highly specific and varies across species, influencing the behavioral outcomes of peptide administration in laboratory settings.
Gq/11 Coupling and Calcium Mobilization
Upon binding to the OXTR, the receptor typically couples to the Gq/11 protein. This interaction triggers a signaling cascade involving phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG).
- IP3 Pathway: Leads to the release of intracellular calcium (Ca2+) from the endoplasmic reticulum.
- DAG Pathway: Activates protein kinase C (PKC).
In neuronal models, this elevation in intracellular calcium is crucial for increasing neuronal excitability and facilitating neurotransmission within sexual arousal circuits.
Neural Circuitry: The Hypothalamic Control of Arousal
Research suggests that oxytocin acts as a central integrator of sexual signals, particularly within the hypothalamus and limbic system. The paraventricular nucleus (PVN) is identified as a primary site of integration.
The PVN-Spinal Cord Axis
In male rodent models, studies demonstrate that parvocellular oxytocinergic neurons in the PVN project directly to the spinal cord. These projections regulate the autonomic outflow necessary for penile erection. Activation of these neurons has been observed to facilitate pro-erectile responses, whereas lesions in the PVN can disrupt these reflexes.
The Medial Preoptic Area (MPOA)
The MPOA is another critical region rich in OXTRs. Research indicates that extracellular dopamine levels in the MPOA increase during sexual activity. Oxytocin appears to modulate this dopaminergic activity, creating a feedback loop that enhances sexual motivation and copulatory performance in experimental subjects.
Interaction with Neurotransmitter Systems
Oxytocin does not function in isolation; rather, it acts as a neuromodulator, interacting with several other neurotransmitter systems to regulate sexual behavior.
The Dopamine Synergy
One of the most heavily researched interactions is between oxytocin and dopamine.
- Ventral Tegmental Area (VTA): Oxytocin neurons project to the VTA, a key component of the brain's reward system.
- Nucleus Accumbens: Oxytocin signaling in the nucleus accumbens has been linked to the rewarding properties of sexual behavior.
- Mechanism: Research suggests that OXT facilitates dopamine release, thereby reinforcing sexual motivation and the maintenance of arousal in animal models.
Nitric Oxide (NO) Mediation
In the context of erectile physiology research, oxytocin has been shown to activate nitric oxide synthase (NOS). This enzyme mediates the production of nitric oxide, a potent vasodilator. Central administration of oxytocin in laboratory rats has been observed to increase intracavernous pressure, an effect often blocked by NOS inhibitors, suggesting that NO is a downstream mediator of central oxytocinergic pro-erectile effects.
Sexual Dimorphism in Oxytocin Research
Peptide researchers must account for significant sexual dimorphism in oxytocin signaling. While the mechanisms discussed above largely pertain to male physiology, the role of OXT in female models differs structurally and functionally.
- Lordosis Behavior: In female rodents, oxytocin facilitates lordosis (receptive posture) through interactions with estrogen and progesterone receptors in the ventromedial hypothalamus (VMH).
- Receptor Density: Estrogen has been shown to upregulate OXTR expression in specific brain regions, sensitizing the subject to the effects of endogenous or exogenous oxytocin during specific phases of the estrous cycle.
Future Directions in Peptide Development
Current investigations are moving beyond simple administration of native oxytocin. The short half-life of the native peptide limits its utility in certain long-duration studies. Consequently, research is shifting toward:
- Analogs: Developing stable oxytocin analogs with higher selectivity for the OXTR over vasopressin receptors.
- Blood-Brain Barrier (BBB) Penetration: Investigating delivery vectors that allow peptides to cross the BBB more effectively to target central mechanisms.
- Antagonists: Using selective OXTR antagonists to dissect the precise contribution of oxytocin to specific phases of the sexual response cycle.
Conclusion
The body of evidence supports oxytocin as a fundamental neuropeptide in the regulation of sexual arousal pathways. Through its interactions with dopamine, modulation of spinal autonomic reflexes, and activation of intracellular calcium signaling, OXT serves as a potent central mediator of sexual function in preclinical models. For researchers at SPARX BIOTECH PEPTIDE, understanding these specific pathways is essential for the development of targeted experimental protocols and the advancement of neuroendocrinology.