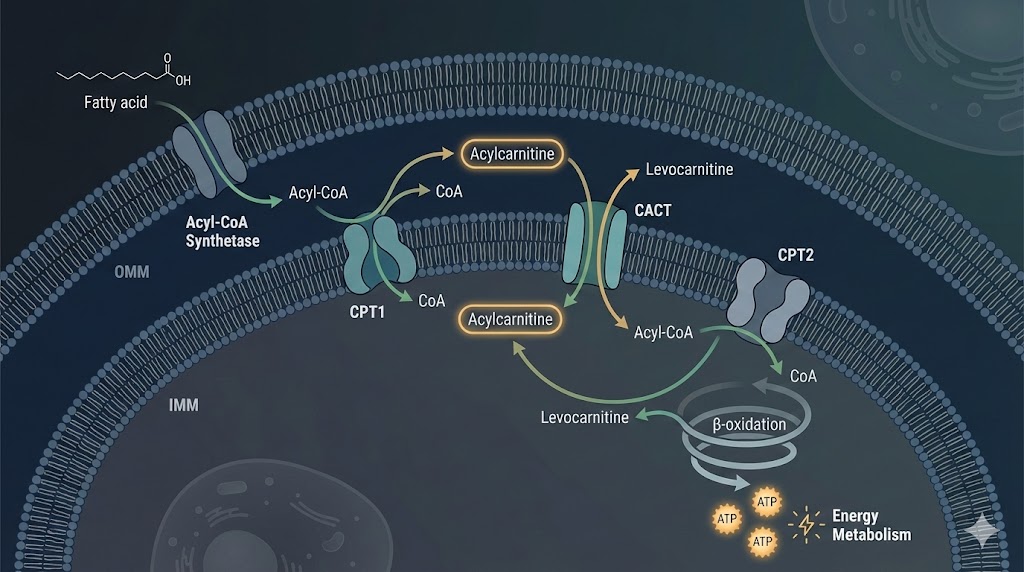
In the field of metabolic biochemistry and cellular physiology, few mechanisms are as critical to bioenergetics as the transport of lipids into the mitochondria. Levocarnitine (L-carnitine) is a naturally occurring quaternary ammonium compound that plays an obligate role in this process. For researchers investigating metabolic flux, mitochondrial efficiency, or lipid peroxidation, understanding the precise molecular dynamics of Levocarnitine is fundamental.
SPARX BIOTECH PEPTIDE presents this technical overview to elucidate the biochemical pathway known as the "carnitine shuttle." This system allows long-chain fatty acids—which are otherwise impermeable to the inner mitochondrial membrane—to traverse the barrier and enter the matrix for β-oxidation. This article explores the enzymatic steps, regulatory points, and current research implications of Levocarnitine in a laboratory setting.
The Mitochondrial Barrier: A Bioenergetic Challenge
The mitochondrion is defined by its double-membrane structure. While the outer mitochondrial membrane contains porins (VDACs) that allow the passage of small molecules and ions, the inner mitochondrial membrane (IMM) is highly impermeable. This impermeability is essential for maintaining the electrochemical proton gradient (Δψm) required for ATP synthesis, but it presents a logistical challenge for substrate transport.
Short- and medium-chain fatty acids can diffuse freely into the mitochondria. However, long-chain fatty acids (LCFAs), such as palmitic acid, cannot cross the IMM unassisted. In research models focusing on high-energy tissues, the inability to transport LCFAs results in a cessation of β-oxidation and cellular energy failure. Levocarnitine acts as the essential carrier molecule in the transport system evolved to solve this permeability problem.
The Carnitine Shuttle System: A Biochemical Pathway
The transport of LCFAs involves a sequential enzymatic process involving Levocarnitine and three key mitochondrial proteins. This system is collectively termed the "carnitine shuttle."
1. Activation of Fatty Acids
Before interaction with Levocarnitine, free fatty acids in the cytosol must be "activated." This is catalyzed by the enzyme acyl-CoA synthetase (also known as thiokinase), located on the outer mitochondrial membrane. The reaction consumes ATP to attach a Coenzyme A (CoA) moiety to the fatty acid, forming Long-Chain Acyl-CoA.
2. The Rate-Limiting Step: CPT1
The first dedicated step of the shuttle is catalyzed by Carnitine Palmitoyltransferase 1 (CPT1). This enzyme is embedded in the outer mitochondrial membrane. CPT1 catalyzes the transfer of the acyl group from CoA to Levocarnitine, forming Acylcarnitine and releasing free CoA back into the cytosol.
This step is critical in metabolic research because CPT1 is the primary regulatory site for fatty acid oxidation. It is allosterically inhibited by Malonyl-CoA (an intermediate of fatty acid synthesis), ensuring that synthesis and degradation do not occur simultaneously.
3. Translocation via CACT
Once formed, the Acylcarnitine molecule is transported across the inner mitochondrial membrane by the Carnitine-Acylcarnitine Translocase (CACT). This is an antiporter mechanism: as one molecule of Acylcarnitine enters the matrix, one molecule of free Levocarnitine is exported back to the intermembrane space. This stoichiometric exchange ensures a continuous supply of cytosolic Levocarnitine for further transport cycles.
4. Reformation via CPT2
Upon reaching the mitochondrial matrix, the acyl group must be transferred back to a CoA molecule to enter the β-oxidation spiral. This reaction is catalyzed by Carnitine Palmitoyltransferase 2 (CPT2), located on the inner face of the inner mitochondrial membrane.
The regenerated Acyl-CoA is now available for oxidation, and the free Levocarnitine is returned to the cytosol by CACT.
Levocarnitine in Metabolic Research Models
In biotechnology and peptide research, Levocarnitine is frequently utilized as a variable to study mitochondrial dynamics. It serves as a vital tool in in vitro and in vivo preclinical models.
Investigating Oxidative Stress
Mitochondria are the primary source of Reactive Oxygen Species (ROS) within the cell. Research suggests that when fatty acid transport is inefficient, accumulation of cytosolic lipids can lead to lipotoxicity and increased oxidative stress. Studies utilizing Levocarnitine often aim to observe its influence on ROS scavenging capabilities and membrane stability under hypoxic or ischemic conditions.
Metabolic Flux Analysis
Researchers employing metabolic flux analysis often manipulate the concentration of available Levocarnitine to study the kinetics of the CPT1 enzyme. By altering the availability of the carrier molecule, scientists can quantify the maximum capacity of the mitochondrial oxidative phosphorylation system (OXPHOS) in various cell lines, such as cardiomyocytes or hepatocytes.
Interaction with Peptides
In the context of peptide synthesis and application, Levocarnitine is often studied alongside mitochondrial-targeted peptides (e.g., SS-31 or MOTS-c). The objective in these research settings is to determine if synergistic mechanisms exist that can enhance mitochondrial biogenesis or efficiency. For example, does the presence of specific signaling peptides alter the expression of CPT1, thereby increasing the demand for Levocarnitine transport?
The Importance of Research-Grade Purity
In experimental settings, the integrity of data relies heavily on the purity of the reagents used. Impurities in Levocarnitine or related metabolic substrates can inhibit enzymatic function (particularly CPT1) or introduce confounding variables in mass spectrometry analysis.
High-purity, research-grade Levocarnitine ensures that observed effects on fatty acid oxidation rates are solely attributable to the molecule's mechanism of action as a transport carrier, rather than exogenous contaminants.
Future Directions in Mitochondrial Biology
The study of Levocarnitine and the carnitine shuttle remains a vibrant area of investigation. Current research trends focus on:
- Genetic Polymorphisms: Investigating how variances in CPT1/CPT2 genes affect transport efficiency in cell models.
- Metabolic Flexibility: Understanding how cells switch between glucose and fatty acid oxidation (the Randle Cycle) in the presence of varying Levocarnitine concentrations.
- Aging Models: Examining the decline of mitochondrial transport efficiency in senescent cell lines and the potential role of substrate availability.
Summary
Levocarnitine is not merely a supplement; it is an obligate biochemical cofactor required for the movement of energy-rich lipids into the mitochondrial furnace. From the initial activation by Acyl-CoA Synthetase to the final regeneration by CPT2, the carnitine shuttle represents an elegant solution to the challenge of membrane permeability. For researchers at SPARX BIOTECH PEPTIDE, understanding this pathway is essential for advancing the frontiers of metabolic science and peptide utility.