In the field of biotechnology and peptide research, IGF-1 LR3 (Long Arg3 Insulin-like Growth Factor-I) has emerged as a compound of significant interest due to its potent anabolic properties. Unlike endogenous insulin-like growth factor-1 (IGF-1), which has a rapid turnover rate, IGF-1 LR3 is a modified analog designed to exhibit extended stability and distinct biological activity.
Central to its investigation is its capacity to influence nitrogen retention—a critical biomarker for protein synthesis and muscle hypertrophy. Positive nitrogen balance indicates that the rate of protein synthesis exceeds the rate of protein breakdown, a state essential for tissue repair and growth. This article explores the biochemical mechanisms through which IGF-1 LR3 modulates nitrogen retention, its interaction with the mTOR signaling pathway, and its observed effects on skeletal muscle cellular dynamics.
Structural Modifications and Bioavailability
To understand the potency of IGF-1 LR3, one must first examine its structural engineering. Native IGF-1 is a polypeptide hormone consisting of 70 amino acids. Its biological activity in the bloodstream is tightly regulated by Insulin-like Growth Factor Binding Proteins (IGFBPs). These proteins bind to IGF-1, inhibiting its interaction with cell surface receptors and rapidly clearing it from circulation—typically resulting in a half-life ($t_0.5$) of less than 20 minutes.
IGF-1 LR3 differs from native IGF-1 in two key ways:
- Substitution: The Glutamic acid at position 3 is replaced with Arginine (Arg3).
- Extension: A 13-amino acid extension is added to the N-terminus (the 'Long' portion).
Research indicates that these modifications drastically reduce the peptide's affinity for IGFBPs. Because it does not bind strongly to these inhibiting proteins, IGF-1 LR3 remains biologically active in the system for a significantly longer duration, with a half-life estimated between 20 to 30 hours in preclinical models. This extended bioavailability allows for sustained receptor activation, which is a primary driver of its enhanced effects on nitrogen retention compared to its endogenous counterpart.
Mechanisms of Action: The Nitrogen Retention Pathway
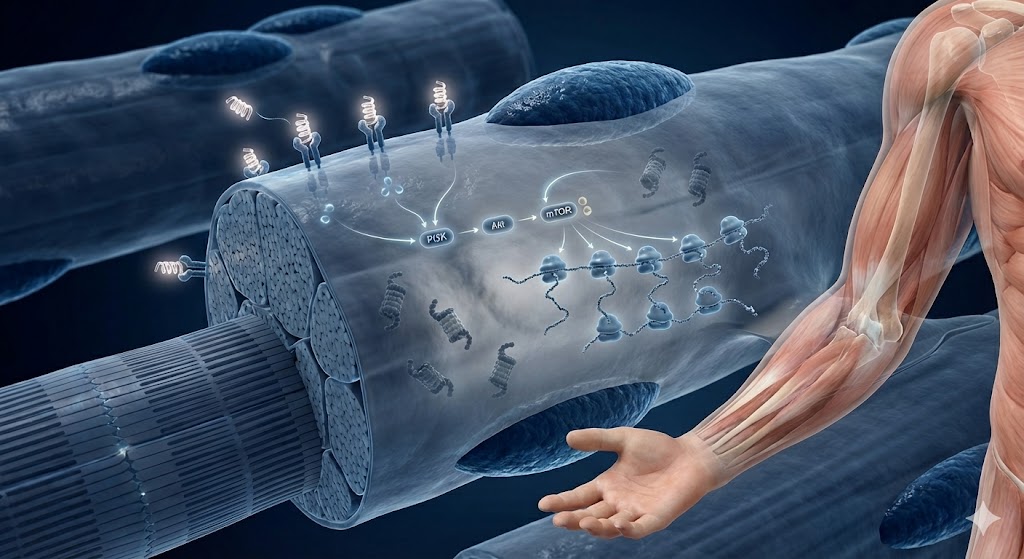
Nitrogen retention in skeletal muscle is not a passive process; it is the metabolic result of upregulated protein synthesis and downregulated proteolysis (protein breakdown). Research suggests that IGF-1 LR3 drives this anabolic state primarily through the PI3K/Akt/mTOR signaling axis.
1. Activation of the PI3K/Akt/mTOR Axis
When IGF-1 LR3 binds to the IGF-1 Receptor (IGF-1R) on the surface of myoblasts (muscle cells), it triggers the autophosphorylation of the receptor. This recruits Instulin Receptor Substrate-1 (IRS-1), which subsequently activates Phosphoinositide 3-kinase (PI3K).
The activation of PI3K leads to the phosphorylation of Akt (Protein Kinase B). Akt is a 'master switch' for cellular growth that directly activates the Mammalian Target of Rapamycin (mTOR). The mTOR complex is widely recognized in scientific literature as the central regulator of protein synthesis. Once active, mTOR signals downstream effectors—specifically p70S6K (ribosomal protein S6 kinase) and 4E-BP1 (eukaryotic translation initiation factor 4E-binding protein 1)—to initiate the translation of mRNA into new muscle proteins.
2. Inhibition of Catabolic Pathways
Nitrogen retention is maximized not just by building protein, but by preventing its loss. Studies suggest that the Akt pathway activated by IGF-1 LR3 also phosphorylates FoxO transcription factors. Phosphorylated FoxO is sequestered in the cytoplasm, preventing it from entering the nucleus and transcribing 'atrogins'—genes responsible for muscle atrophy (such as MuRF1 and Atrogin-1). By inhibiting these ubiquitin-proteasome pathways, IGF-1 LR3 may effectively reduce the rate of muscle protein breakdown.
Cellular Impact on Skeletal Muscle
Beyond the biochemical pathways of protein synthesis, IGF-1 LR3 has been investigated for its influence on the cellular machinery of muscle tissue, specifically regarding satellite cells and hyperplasia.
Satellite Cell Proliferation and Differentiation
Skeletal muscle fibers are post-mitotic, meaning they do not divide. Muscle growth and repair rely on a population of stem cells known as satellite cells located on the periphery of the muscle fiber.
Research demonstrates that IGF-1 LR3 is a potent mitogen for these cells. Upon activation, satellite cells proliferate (divide) and differentiate into myoblasts. These myoblasts can then fuse with existing muscle fibers to donate their nuclei. Since the capacity of a muscle fiber to synthesize protein depends on its number of myonuclei (the 'myonuclear domain' theory), the addition of new nuclei is a critical step in long-term, sustainable hypertrophy.
Hyperplasia vs. Hypertrophy
While hypertrophy refers to the increase in the size of existing muscle fibers, hyperplasia refers to the splitting of fibers to create new ones. While hyperplasia is difficult to achieve in adult organisms, preclinical studies involving potent IGF-1 analogs suggest that high levels of localized IGF-1 signaling may encourage fiber splitting and new fiber formation, contributing to an overall increase in muscle density and nitrogen-retentive capacity.
Comparative Research: IGF-1 LR3 vs. Native IGF-1
The distinction between IGF-1 LR3 and native IGF-1 is a frequent subject of comparative analysis. In vitro studies using L6 myoblasts (a rat skeletal muscle cell line) have often shown that IGF-1 LR3 exhibits higher potency than native IGF-1.
| Feature | Native IGF-1 | IGF-1 LR3 |
|---|---|---|
| Half-Life | < 20 Minutes | 20–30 Hours |
| IGFBP Affinity | High (inhibitory) | Very Low (active) |
| Primary Action | Systemic growth regulation | Sustained local anabolic signaling |
| Potency | Standard | Enhanced (due to stability) |
The lack of IGFBP binding means that nearly all the administered IGF-1 LR3 is available to bind to receptors. In contrast, a significant portion of exogenous native IGF-1 would be immediately neutralized by circulating binding proteins.
Future Directions in Peptide Research
The ability of IGF-1 LR3 to promote nitrogen retention and inhibit catabolism makes it a molecule of significant interest for future therapeutic research. Scientists are currently investigating its potential applications in conditions characterized by muscle wasting, such as sarcopenia (age-related muscle loss), cachexia (wasting syndrome often seen in cancer), and recovery from severe burns or immobilization.
Current research is focused on optimizing stability and understanding the long-term effects of chronic receptor stimulation. As biotechnology advances, the precise mapping of how IGF-1 LR3 influences gene expression in skeletal muscle continues to evolve, offering new insights into the fundamental biology of muscle growth.