Introduction to Bremelanotide (PT-141)
Bremelanotide, widely referenced in laboratory settings by its developmental code PT-141, is a synthetic peptide originally developed from the hormone Melanotan II. It is a cyclic heptapeptide lactam analogue of alpha-melanocyte-stimulating hormone (α-MSH).
Unlike many peptides that primarily influence the vascular system, Bremelanotide research has focused heavily on its ability to act directly on the central nervous system (CNS). By traversing the blood-brain barrier, this peptide allows researchers to investigate the complex signaling pathways involved in physiological arousal and neural modulation. As a research-grade compound, it serves as a critical tool for laboratory investigations into the melanocortin system, specifically how receptor activation influences hypothalamic function.
Mechanism of Action: Targeting Melanocortin Receptors
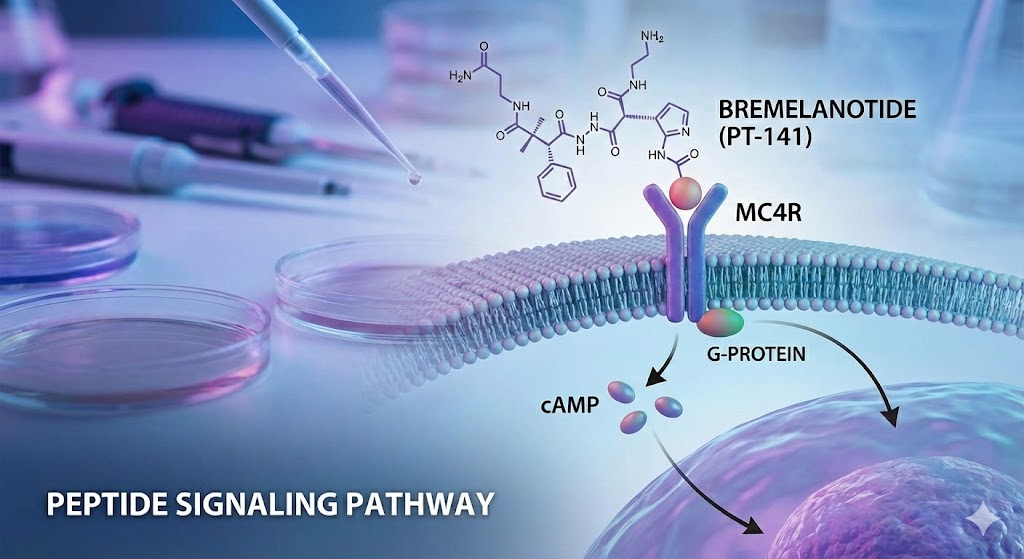
The primary scientific interest in Bremelanotide lies in its profile as a non-selective melanocortin receptor agonist. The melanocortin system is a key regulator of various physiological functions, including energy homeostasis, inflammation, and sexual behavior.
Specificity for MC3R and MC4R
Research indicates that Bremelanotide exhibits high affinity for specific subtypes of melanocortin receptors. While α-MSH activates MC1R, MC3R, MC4R, and MC5R, Bremelanotide has shown a distinct binding preference in preclinical assays:
- MC4R (Melanocortin 4 Receptor): This is the primary target of investigation regarding CNS-mediated arousal. The MC4R is highly expressed in the hypothalamus, particularly in the paraventricular nucleus. Activation of this receptor is hypothesized to trigger downstream signaling cascades relevant to metabolic and behavioral research.
- MC3R (Melanocortin 3 Receptor): Bremelanotide also demonstrates agonist activity at MC3R, which is associated with energy balance and feed efficiency in animal models.
Signal Transduction and cAMP Pathways
Upon binding to G-protein-coupled melanocortin receptors, Bremelanotide is observed to initiate intracellular signaling via the cyclic adenosine monophosphate (cAMP) pathway. In in vitro cultures, the binding event typically stimulates adenylate cyclase, leading to an increase in intracellular cAMP levels. This cascade is a fundamental area of study for biochemists seeking to understand how peptide ligands translate extracellular binding into physiological responses within neurons.
Investigating Central Nervous System Modulation
One of the defining characteristics of PT-141 peptide mechanism studies is the investigation of CNS-mediated pathways independent of the vascular system. Earlier research into sexual function often focused on phosphodiesterase type 5 (PDE5) inhibitors, which act on the vascular system to increase blood flow. In contrast, Bremelanotide allows scientists to explore the neurological basis of arousal.
Hypothalamic Activation in Animal Models
Preclinical studies, primarily involving rodent models, have utilized Bremelanotide to map neural circuits. When administered in controlled laboratory settings, the peptide has been observed to induce immediate early gene expression (such as c-Fos) in the paraventricular nucleus of the hypothalamus. These studies provide data on how chemical signals in the brain modulate complex behaviors, differentiating central processing from peripheral physiological reflexes.
Dopamine Interaction
Further research suggests a potential interplay between melanocortin activation and the dopaminergic system. Neuropeptide signaling research involving Bremelanotide investigates whether MC4R activation modulates dopamine release in the medial preoptic area (mPOA), a region critical for motivation and reward processing. This cross-talk between peptide and neurotransmitter systems remains a vital area of neuroendocrinology.
Comparative Analysis: Bremelanotide vs. Melanotan II
In the context of peptide synthesis and categorization, it is essential to distinguish Bremelanotide from its parent compound, Melanotan II.
- Chemical Structure: While both are cyclic analogues of α-MSH, Bremelanotide possesses a C-terminal acid function, whereas Melanotan II has a C-terminal amide. This structural modification is significant in structure-activity relationship (SAR) studies.
- Receptor Selectivity: Melanotan II is known for potent activity at the MC1R (associated with pigmentation). Bremelanotide was developed to minimize activity at MC1R while retaining high affinity for MC4R. This makes Bremelanotide a more specific tool for researchers aiming to isolate CNS effects without the confounding variable of melanogenesis (pigmentation induction) often seen with Melanotan II.
Broad Applications in Biochemical Research
Beyond behavioral studies, the versatility of melanocortin receptor agonists like Bremelanotide allows for diverse applications in cellular biology and physiology.
Inflammation and Ischemia
The melanocortin system is increasingly recognized for its anti-inflammatory properties. Experimental models of ischemia-reperfusion injury have utilized agonists like Bremelanotide to assess potential cytoprotective effects. Unlike peptides studied in tissue regeneration models like GHK-Cu, Bremelanotide's influence is primarily mediated through the modulation of pro-inflammatory cytokines, offering a pathway for studying immune response modulation in non-clinical settings.
Metabolic Research
Given the role of MC3R and MC4R in energy homeostasis, Bremelanotide is occasionally employed in metabolic research. Scientists investigate how receptor activation influences food intake and energy expenditure in obesity models, similar to studies involving MOTS-c and mitochondrial metabolism.
Future Directions in Peptide Development
The study of Bremelanotide continues to evolve as analytical techniques improve. Current trends in peptide synthesis RUO (Research Use Only) focus on improving the stability and bioavailability of such neuropeptides in experimental solvents.
Researchers are also exploring biased signaling, where modified peptide analogues might selectively activate specific intracellular pathways downstream of the MC4R. This level of precision aims to decouple desirable physiological probes from potential off-target effects in cell culture systems.
Conclusion
Bremelanotide (PT-141) remains a cornerstone in the study of the melanocortin system. By acting as a potent agonist for MC3R and MC4R, it provides a window into the central regulation of physiological function, distinct from vascular mechanisms. For the scientific community, this peptide offers a robust platform for investigating the complex interplay between neuropeptides, receptor kinetics, and neural circuitry.