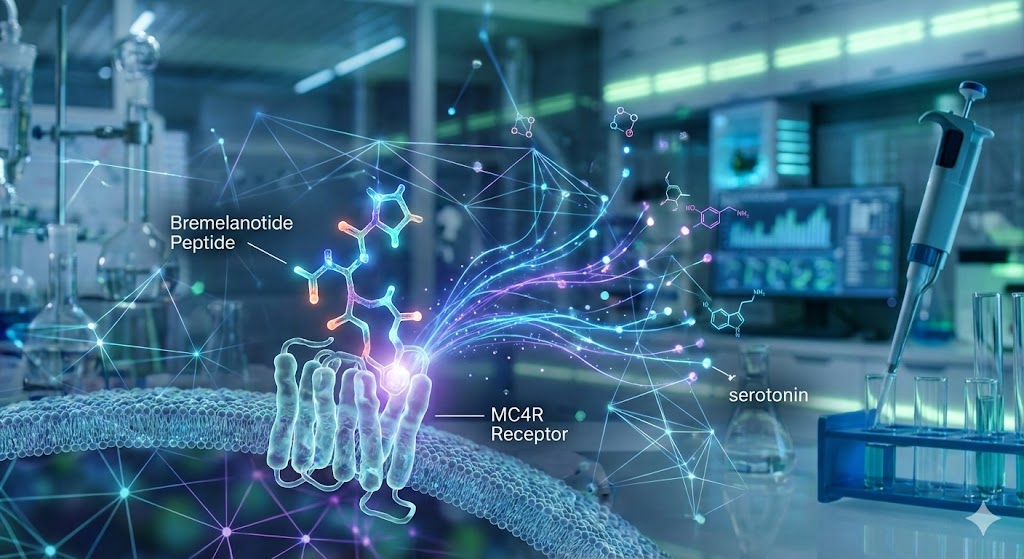
Bremelanotide, a synthetic peptide analog of alpha-melanocyte-stimulating hormone (α-MSH), has emerged as a significant compound in the study of neurobiology and sexual function. Unlike traditional agents that target peripheral vascular mechanics, Bremelanotide is investigated for its ability to modulate central nervous system (CNS) pathways.
Current research focuses on its interaction with melanocortin receptors, specifically the melanocortin-4 receptor (MC4R), and its potential role in regulating the neurochemical cascades associated with sexual desire and arousal.
The Neurobiology of Sexual Desire: A Central Mechanism
Sexual behavior is a complex physiological process regulated by a delicate balance of excitatory and inhibitory signals within the brain. Research suggests that sexual desire is not merely a vascular event but a cognitive and neurochemical phenomenon rooted in the limbic system and hypothalamus.
The Role of Melanocortin Receptors
Melanocortins are a family of neuropeptides that bind to five distinct receptors (MC1R through MC5R). Scientific literature identifies MC4R as a critical modulator of energy homeostasis and sexual behavior.
Bremelanotide acts as a non-selective agonist with high affinity for MC4R. When activated, these receptors—located densely in the Medial Preoptic Area (mPOA) of the hypothalamus—are believed to trigger downstream signaling pathways that influence sexual motivation. The mPOA is often cited in neurobiological studies as a primary integration center for sexual stimuli.
Dopamine and Serotonin Modulation
The regulation of sexual desire involves an interplay between key neurotransmitters:
- Dopamine: Often described as the primary driver of reward and motivation. Preclinical studies investigate whether MC4R activation by Bremelanotide enhances extracellular dopamine levels in the mPOA, potentially amplifying the "excitatory" signals of desire.
- Serotonin: Generally acts as an inhibitory force in sexual regulation. Research explores whether Bremelanotide may help modulate this inhibition, shifting the balance in favor of arousal.
Preclinical Research: Bremelanotide in Animal Models
Much of the foundational knowledge regarding Bremelanotide comes from controlled animal studies designed to map its mechanism of action. These "Research Use Only" investigations provide insight into how the peptide functions in the absence of psychological or social variables found in human subjects.
Investigating "Solicitation" Behavior
In rodent models, sexual desire is often operationalized as "solicitation" behavior—active measures taken by the subject to initiate contact.
- Key Findings: Studies utilizing ovariectomized, hormone-primed rats have observed that administration of Bremelanotide significantly increased solicitation behaviors.
- Interpretation: Researchers hypothesize that this behavioral change indicates a direct effect on the appetitive (motivational) phase of sexual function, rather than the consummatory (physical) phase.
fMRI and Brain Imaging Studies
Advanced neuroimaging techniques, such as functional Magnetic Resonance Imaging (fMRI), have been employed to visualize brain activity following Bremelanotide administration.
- Observed Activity: Research indicates that MC4R agonism may alter blood-oxygen-level-dependent (BOLD) signals in regions associated with reward and sensory processing.
- Functional Connectivity: Some studies suggest enhanced connectivity between the amygdala and other limbic structures, potentially facilitating the processing of erotic or excitatory stimuli.
Distinct from PDE5 Inhibitors
A critical distinction in peptide research is the difference between central and peripheral mechanisms.
- Peripheral Agents (e.g., PDE5 Inhibitors): Compounds like Sildenafil work primarily on the vascular system, enhancing nitric oxide pathways to improve blood flow to genital tissue. They do not directly influence sexual desire or CNS signaling.
- Central Agents (e.g., Bremelanotide): Research positions Bremelanotide as a CNS-acting agent. It does not rely on vasodilation as a primary mechanism. Instead, it is studied for its ability to "switch on" the neural circuits of desire, regardless of local blood flow dynamics.
This distinction makes Bremelanotide a unique subject of study for researchers interested in the psychogenic and neurochemical aspects of sexual dysfunction, rather than purely mechanical impairments.
Future Directions in Melanocortin Research
The scientific community continues to explore the broader applications of MC4R agonists. While current focus remains on sexual desire regulation, ongoing research aims to elucidate:
- Receptor Specificity: Developing analogs with higher selectivity for MC4R to minimize off-target effects (such as interaction with MC1R, involved in pigmentation).
- Neuroplasticity: Investigating whether long-term modulation of melanocortin pathways can induce lasting changes in neural circuitry related to motivation.
- Synergistic Mechanisms: Exploring how melanocortin signaling interacts with other peptide systems, such as oxytocin and kisspeptin, to regulate complex social and sexual behaviors.
Summary of Research Status
- Compound: Bremelanotide (PT-141)
- Class: Cyclic heptapeptide lactam analog of α-MSH.
- Primary Target: Melanocortin-4 Receptor (MC4R) agonist.
- Primary Research Interest: Central nervous system regulation of sexual desire and motivation.