Obesity is increasingly understood not merely as a disorder of energy storage, but as a condition of chronic, low-grade inflammation. This intersection of the immune system and metabolic regulation—often termed 'immunometabolism'—has opened new avenues for investigation. Among the small molecules currently under scrutiny is Amlexanox, a compound originally characterized for its anti-allergic properties, which has recently emerged as a significant subject of interest in obesity research due to its ability to inhibit specific inflammatory kinases: TBK1 and IKKε.
This article reviews the scientific literature surrounding Amlexanox, focusing on its mechanism of action, its influence on white adipose tissue browning, and its role in preclinical metabolic models.
The Target: Inflammatory Kinases TBK1 and IKKε
To understand the research potential of Amlexanox, one must first understand the molecular targets it inhibits.
Research indicates that in states of obesity, specific non-canonical IκB kinases—specifically TANK-binding kinase 1 (TBK1) and IκB kinase ε (IKKε)—are markedly upregulated in metabolic tissues, particularly in the liver and adipose (fat) tissue. These kinases are induced by NF-κB activation, a primary regulator of inflammatory immune responses.
While these kinases play a role in the innate immune response (defending against pathogens), their chronic activation in obesity appears to create a maladaptive feedback loop. Scientific data suggests that elevated levels of TBK1 and IKKε contribute to 'catecholamine resistance' in adipocytes. Under normal conditions, catecholamines (like norepinephrine) signal fat cells to burn energy; however, when TBK1/IKKε are elevated, this signaling pathway is dampened, locking the energy within the cell and promoting storage over expenditure.
Mechanism of Action: Restoring Metabolic Flexibility
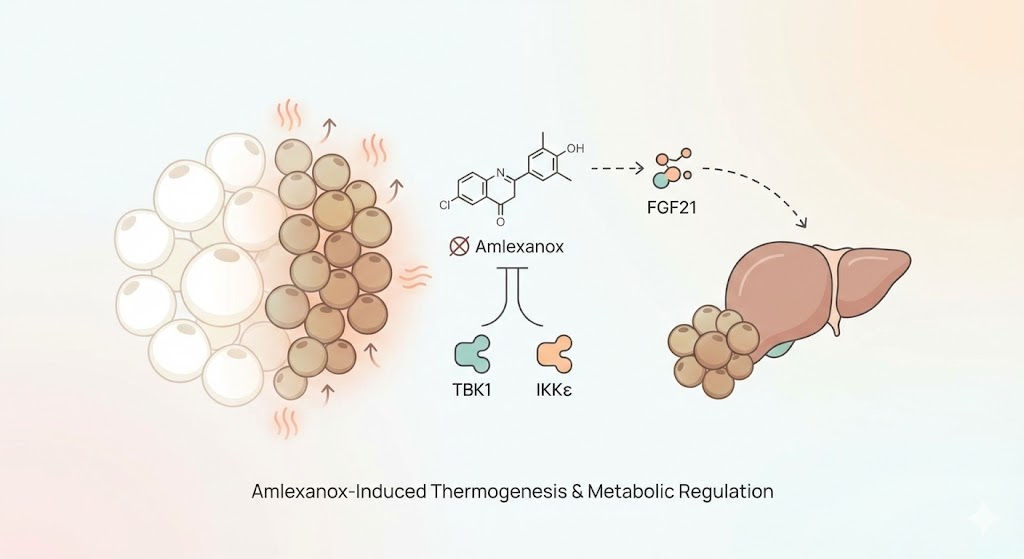
Amlexanox has been identified in high-throughput screenings as a selective inhibitor of both TBK1 and IKKε. By blocking these kinases, Amlexanox is hypothesized to break the link between inflammation and metabolic dysfunction. Current research focuses on three primary mechanisms:
1. Promoting the Browning of White Adipose Tissue
Perhaps the most significant finding in Amlexanox research is its potential to induce 'browning' or 'beiging' of white adipose tissue (WAT). White fat primarily stores energy, while brown fat dissipates energy as heat (thermogenesis).
Inhibition of TBK1 and IKKε has been observed to increase the expression of Uncoupling Protein 1 (UCP1), a mitochondrial protein essential for thermogenesis. This process allows white adipocytes to adopt the metabolic characteristics of brown fat, thereby increasing total energy expenditure without a change in physical activity.
2. Enhancing Catecholamine Sensitivity
As noted, obesity-induced inflammation can render fat cells resistant to hormonal signals. Research in murine models suggests that Amlexanox administration may restore the sensitivity of adipocytes to beta-adrenergic stimulation. This restoration allows the cells to respond effectively to lipolytic (fat-burning) signals, releasing stored fatty acids to be oxidized for energy.
3. The FGF21 Pathway
Recent studies have highlighted the role of Fibroblast Growth Factor 21 (FGF21) in the efficacy of Amlexanox. Inhibition of IKKε and TBK1 appears to stimulate the secretion of FGF21, a peptide hormone that regulates glucose and lipid metabolism. FGF21 is known to improve insulin sensitivity and promote energy expenditure, suggesting that Amlexanox may act as an upstream regulator of this critical metabolic hormone.
Findings in Preclinical Metabolic Models
The majority of data regarding Amlexanox in a metabolic context is derived from rodent models of diet-induced obesity (DIO) and genetic obesity (ob/ob mice).
- Weight Loss: In several studies, obese mice treated with Amlexanox exhibited significant weight loss compared to controls, despite maintaining similar food intake. This weight loss was attributed primarily to increased energy expenditure via thermogenesis.
- Insulin Sensitization: Amlexanox treatment in diverse mouse models has been correlated with improved glucose tolerance and insulin sensitivity. Researchers observed reduced fasting insulin levels and improved clearance of blood glucose.
- Reduction of Hepatic Steatosis: Fatty liver is a common comorbidity of obesity. Preclinical investigations have noted that inhibiting TBK1/IKKε reduced the accumulation of triglycerides in the liver, suggesting a potential hepatoprotective mechanism linked to reduced local inflammation.
It is important to note that these effects were observed specifically in the context of obesity-induced inflammation. In lean mice, where TBK1 and IKKε levels are low, the administration of Amlexanox often showed negligible metabolic effects, reinforcing the theory that the molecule acts by normalizing a dysregulated inflammatory state.
The Future of Immunometabolism Research
The investigation of Amlexanox highlights a shifting paradigm in biotechnology: the move away from appetite suppression and toward 'energy expenditure' strategies. By targeting the inflammatory brakes that limit metabolic efficiency, researchers hope to uncover new methods for managing metabolic homeostasis.
While Amlexanox is an approved drug for other indications in certain countries (e.g., for asthma or oral ulcers), its application in metabolic syndrome remains investigational. Current studies are focused on optimizing the delivery, dosage, and chemical structure of TBK1/IKKε inhibitors to maximize their selectivity and potency for research purposes.
For the scientific community, Amlexanox serves as a vital tool compound—a 'probe' that allows researchers to dissect the complex interplay between the immune system and adipose tissue function.
Key Takeaways for Researchers
- Primary Targets: TBK1 and IKKε (non-canonical IκB kinases).
- Primary Mechanism: Reversal of obesity-induced catecholamine resistance.
- Phenotypic Outcome: Increased thermogenesis and browning of white adipose tissue in preclinical models.
- Research Category: Immunometabolism and inflammatory regulation of energy balance.